Update on Ebola Diagnostics at the State and Federal Levels in the U.S.
Clinicians Can Learn Options for Testing Suspected Ebola Virus Infection
December 13, 2019
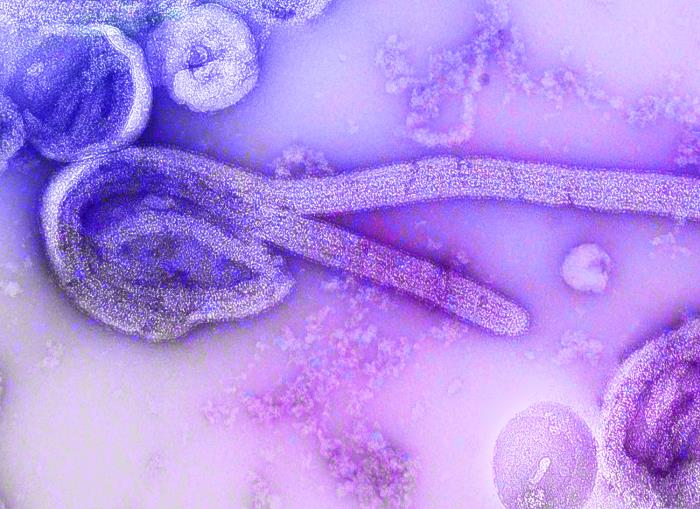
In light of the ongoing Ebola outbreak in the Democratic Republic of the Congo, the Centers for Disease Control and Prevention (CDC) will discuss the current status of Ebola diagnostic testing that is available through the Laboratory Response Network (LRN) and CDC in the U.S. The speakers will also address considerations around the newly FDA-approved rapid diagnostic test (RDT) for Ebola virus infection.
During the CDC Clinician Outreach and Communication Activity (COCA) Call scheduled for Thursday, December 19, 2019 at 2:00 pm–3:00 pm (ET), clinicians will learn about options for testing suspected Ebola virus infection in coordination with state and local public health authorities and CDC, as well as the benefits and limitations of existing diagnostic tools.
Please note: The slides for this presentation are now posted on the call page under the “Call Materials” tab. If you are unable to attend this live COCA Call, the closed-captioned video will also be available to view on-demand on the call page a few days after the call. Free continuing education (CE) is available for this COCA Call. Please visit the call page for instructions on how to receive CE.
More information
COCA Call Page
CDC: Ebola Virus Disease
Thank you for reading AAPA’s News Central
You have 2 articles left this month. Create a free account to read more stories, or become a member for more access to exclusive benefits! Already have an account? Log in.



